✔ 100% Authentic Product
👁️ Currently Viewing 1781
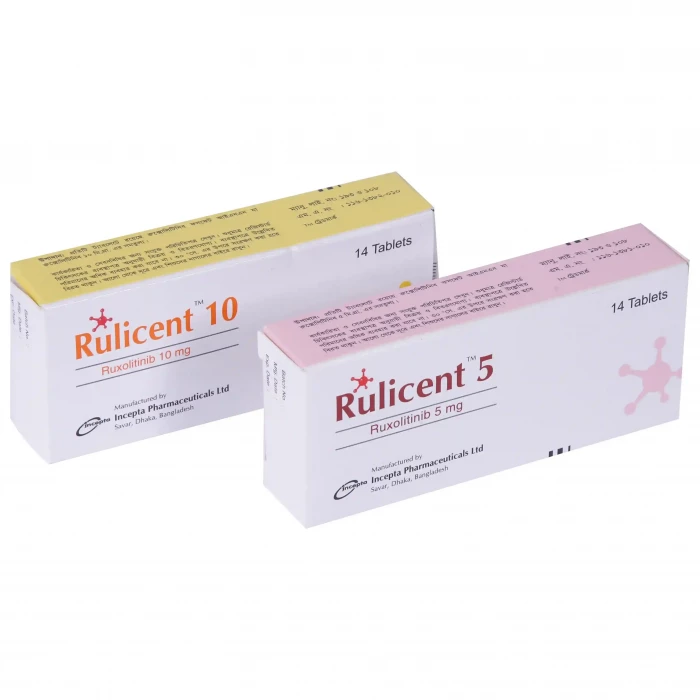
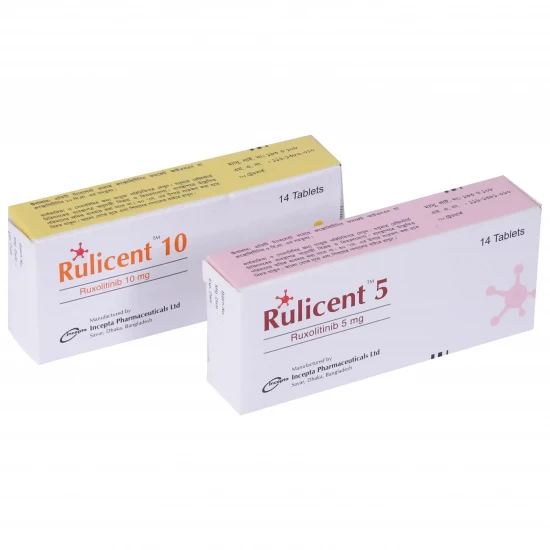
Rulicent 10 | 1 Strip | 7pcs Tablet
Rulicent is approved for the treatment of several hematologic and immune-mediated conditions in adults and selected pediatric populations:
- Myelofibrosis (MF): Rulicent is used in adults diagnosed with intermediate to high-risk MF, including primary myelofibrosis, post-polycythemia vera MF, and post-essential thrombocythemia MF, to manage disease progression and related symptoms.
- Polycythemia Vera (PV): Indicated for adult patients with PV who either do not respond adequately to hydroxyurea or are intolerant to its side effects.
- Steroid-Refractory Acute Graft-versus-Host Disease (GVHD): Approved for adults and pediatric patients aged 12 years and older with acute GVHD that does not respond to corticosteroid treatment.
Pregnancy: There is no conclusive evidence from human studies. Rulicent should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
Breastfeeding: Unknown if excreted in human milk. Discontinue breastfeeding during treatment and for at least two weeks after the last dose due to potential infant exposure.
Discount
Price: ৳ 1,995
MRP:
৳
2100
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Ruxolitinib is an oral selective inhibitor of Janus-associated kinases (JAK1 and JAK2)—enzymes critical for signal transduction from cytokine and growth factor receptors involved in blood cell production and immune regulation.
In myelofibrosis, a condition marked by uncontrolled JAK signaling, Ruxolitinib corrects dysregulation by:
- Suppressing splenomegaly (enlarged spleen)
- Reducing mutant JAK2V617F allele burden
- Lowering circulating inflammatory cytokines (e.g., TNF-α, IL-6)
Pharmacokinetics Overview:
- Absorption: Rapidly absorbed after oral administration; peak levels (Cmax) achieved within 1–2 hours.
- Bioavailability: Oral absorption is at least 95%.
- Distribution: Steady-state volume of distribution ~72 L.
- Plasma Protein Binding: ~97%, primarily to albumin.
- Metabolism: Predominantly via CYP3A4, with minor involvement of CYP2C9.
- Elimination Half-Life: ~3 hours (drug); ~5.8 hours (with metabolites).
- Excretion: Primarily through metabolism—74% in urine, 22% in feces; <1% excreted as unchanged drug.
✔️ Dosage and Administration | Rulicent 10
Route: Oral
Administration: Can be taken with or without food. If a dose is missed, resume with the next scheduled dose without doubling.
For Myelofibrosis (MF):
Dosing is based on baseline platelet count:
≥200 × 10⁹/L: 20 mg twice daily
100–199 × 10⁹/L: 15 mg twice daily
50–99 × 10⁹/L: 5 mg twice daily
Frequent monitoring (CBC every 2–4 weeks) is required to guide dose adjustments.
For Polycythemia Vera (PV):
Starting dose: 10 mg twice daily
Consider reducing to 5 mg twice daily if hemoglobin is between 8–12 g/dL or platelets fall below 75 × 10⁹/L.
For Acute GVHD:
Initial dose: 5 mg twice daily
May increase to 10 mg twice daily after 3 days if ANC and platelet counts remain stable.
- Pediatric Use: Approved for acute GVHD in patients aged 12 years and older. Not established for other indications.
- Elderly: No specific dose adjustments, but greater susceptibility to adverse effects like infections and cytopenias may require cautious use.
✔️ Overdose Management | Rulicent 10
No specific antidote exists.
Single doses up to 200 mg have been tolerated; repeated high doses may cause significant myelosuppression (low WBC, RBC, and platelets).
Supportive measures are recommended. Hemodialysis is ineffective in drug clearance.
✔️ Side Effects | Rulicent 10
Common adverse effects include:
Hematologic: Thrombocytopenia, anemia, neutropenia
General: Headache, dizziness, bruising
Infectious risk: Opportunistic bacterial, fungal, and viral infections
Discontinuation symptoms: Disease rebound, including fever, hypotension, respiratory distress
Skin cancers: Reports of non-melanoma skin cancers (e.g., basal cell, squamous cell, Merkel cell carcinoma)
✔️ Drug Interactions
Fluconazole (>200 mg/day): Inhibits Ruxolitinib metabolism via CYP3A4/CYP2C9, increasing drug levels. Avoid high doses of fluconazole except in acute GVHD.
Strong CYP3A4 Inhibitors (e.g., ketoconazole, itraconazole): Elevate Ruxolitinib exposure. Reduce the Rulicent dose and monitor blood counts closely.
Strong CYP3A4 Inducers: May lower drug levels; frequent monitoring is recommended to adjust dose based on clinical response.
✔️ Contraindications
Patients with known hypersensitivity to Ruxolitinib or any formulation component must avoid this medication.
✔️ Precautions & Warnings
- Bone Marrow Suppression: Monitor CBCs regularly. Thrombocytopenia may require dose reduction or drug interruption. Severe neutropenia is usually reversible upon drug cessation.
- Serious Infections: Delay treatment until active infections are resolved. Monitor throughout therapy.
- Tuberculosis (TB): Screen for TB risk before initiation. Treat latent TB prior to Rulicent initiation if required.
- Progressive Multifocal Leukoencephalopathy (PML): A rare but serious brain infection; discontinue immediately if suspected.
- Herpes Zoster: Educate patients about signs and encourage early medical attention.
- Hepatitis B Reactivation: Regular HBV monitoring is advised in patients with chronic HBV.
- Discontinuation Reactions: Sudden withdrawal may lead to disease flare, systemic symptoms, and serious complications. Taper gradually if feasible.
- Skin Cancer: Conduct periodic skin assessments during long-term use.
- Lipid Monitoring: Check cholesterol and triglyceride levels 8–12 weeks after starting therapy. Manage according to guidelines.
- Store at below 30°C in a dry, light-protected place.
- Keep out of reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.